How Does A Refrigerator Work?
Before we learned how to artificially refrigerate our food and places where we live we used natural ways to bring temperature down. We harvested ice from rivers and lakes in the winter and placed it in ice houses until it was needed in the summer. Then, in 1755, Scottish professor William Cullen showed an experiment that will, slowly but surely, change the world.
Cullen performed a modern version of an ancient method of artificial refrigeration known to ancient Indians and Egyptian - cooling by evaporation. He used a pump to create a partial vacuum in a container where diethyl ether was. This gave diethyl ether lower boiling point and it boiled. Because it started to boil it needed energy to evaporate so it started absorbing heat from the surrounding air lowering temperature of the air. It even produced a small amount of ice. So the artificial refrigeration was born. It wasn’t practical and couldn’t be used for cooling food but it was a start. Others perfected method and, after many experiments, patents and industrial models, in 1915 a practical household refrigerators were introduced.
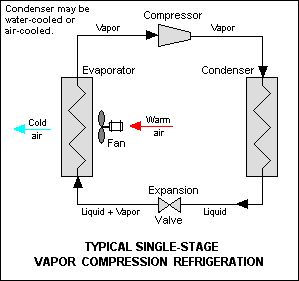
A refrigerator is, basically, a heat engine in which work is done on a refrigerant substance so it could collect energy from a cold region; deliver it in a higher temperature region and with that cooling the cold region even more. Basic elements of a refrigerator are compressor which is connected to the outer, hotter, pipe system (called condenser coils) that is connected to the expansion valve which is connected to the inner, colder, pipe system (evaporator coils) which is connected back to compressor. They all hold refrigerant substance and evaporator coils are placed in a thermally isolated ”box” whose role is to keep its inside cold.
Refrigerant “starts” as a gas (remember - it’s a cycle) at a compressor which rises the pressure which heats the gas. Compressed gas passes through the condenser coils (outer ones), on the back of refrigerator, which are made so the gas will lose the high temperature in them and start turning into a liquid because it is under a high pressure. Liquid refrigerant comes to an expansion valve. Because it’s a cycle, between the valve and the compressor is a low-pressure area - compressor is pulling the liquid refrigerant out of expansion valve into the evaporator coils. Because of the low pressure liquid refrigerant starts boiling and evaporating. Refrigerant, now a gas, goes through evaporator coils and because it needs energy so it could evaporate it “drains” it from the surrounding area and makes it colder. From evaporator coils refrigerant gas goes into compressor and cycle repeats.
Early mechanical refrigeration systems used sulfur dioxide, methyl chloride and ammonia as refrigerants but stopped using sulfur dioxide, methyl chloride because they were toxic. Some other older machines used methyl formate, chloromethane, or dichloromethane. Chlorofluorocarbons were used since the 1950s but were banned since the late 1970s because of the concerns about depletion of the ozone layer. They were substituted with perfluorocarbons and hydrofluorocarbons but they also came under criticism. They are now mostly replaced with fluorinated greenhouse gases.